We’ve reached part 4 of this riveting story of how calcium and other nutrients make it into into the soil and thence into our vegetables and thence into our own bodies (and into chicken eggs). We’ve had some cliffhangers already, so be sure to check out parts one, two and three.
~
4. Solubility, carbonation and chemical weathering
It is the reaction of calcium and calcium compounds with water (see last part) that makes them soluble. Solubility or dissolution is the process by which a “solute†forms a homogeneous mixture with a “solvent†(here water).
This happens as the solute molecule breaks down and its ions dissociate. The positive ions attract the partially-negative oxygen in H2O and the negative ions attract the partially-positive hydrogen in H2O. The ions thus get spread out and become surrounded by the water molecules. The dissolution is complete, or in equilibrium, once it’s all been spread around. (source)
One calcium compound is more soluble than another. Calcium carbonate (our eggshell) has a very poor solubility (47 mg/L at normal atmospheric CO2 partial pressure and 25 degrees C). As we shall see, this is important for gardeners who plan to enrich their soil with eggshell calcium, but I will come to that later.
However, if carbon dioxide is also present, that carbon dioxide will react with the water to form carbonic acid (H2CO3), which is a weak acid – it’s the bubbly in our soft drinks. This carbonic acid will in turn react with the calcium carbonate to form calcium bicarbonate (or calcium hydrogen carbonate). So
CaCO3 + CO2 + H2O → Ca(HCO3)2
Calcium bicarbonate is five times more soluble in water than calcium carbonate—in fact, it exists only in solution.
This is the main process by which carbonate rocks of the Earth’s crust are weathered. As we saw, if water is saturated with carbon dioxide, it produces a mild carbonic acid. This is what happens with (unpolluted) rainwater (water plus atmospheric CO2), which has a pH of around 5.6 (polluted, “acid†rain has a pH of as low as 3.0), and with water in aquifers underground, where it can be exposed to CO2 levels much higher than the ones in the atmosphere.
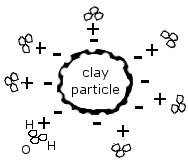
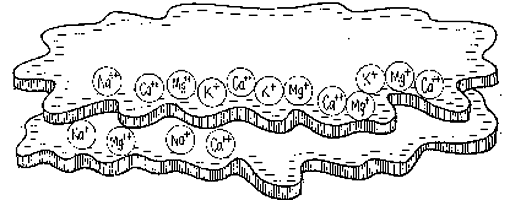
In a process called carbonation, this water’s carbonic acid reacts with the solid calcium carbonate in rocks like limestone or chalk, forming calcium bicarbonate and dissolving it. This solution of water and mineralized calcium is then borne off into the soil, where it is deposited on the colloid, and where it waits to be again dissolved in water and made available to plant roots.
~
Well. That just brings us back to the beginning!